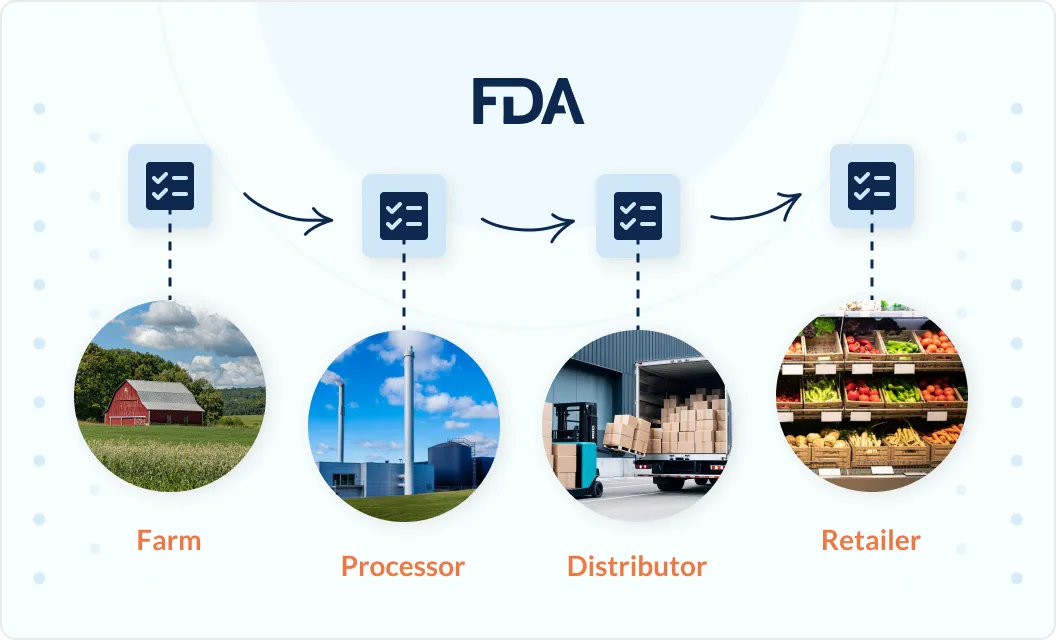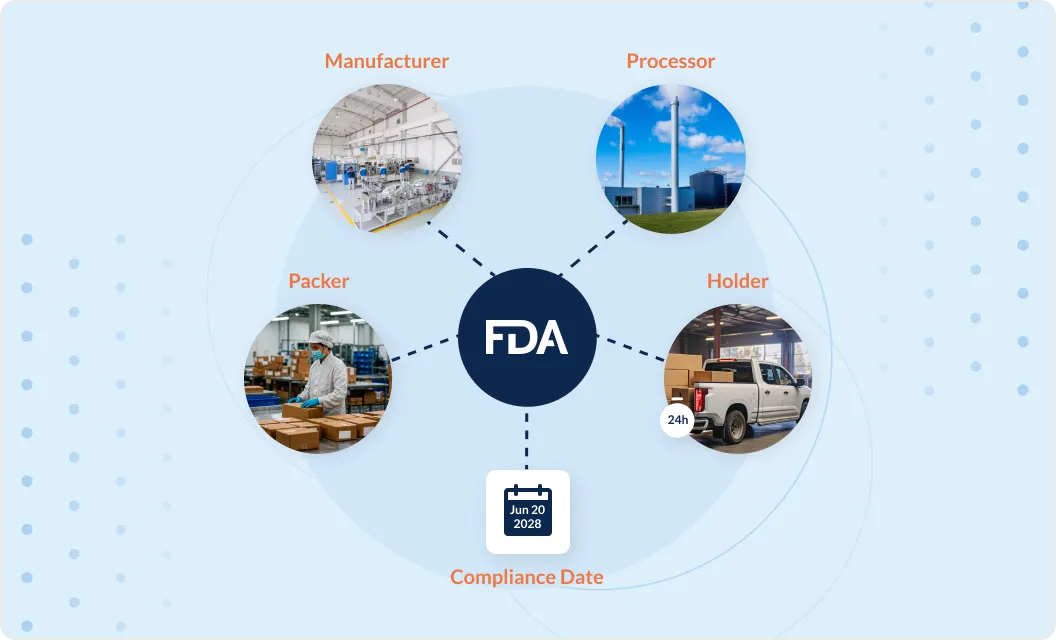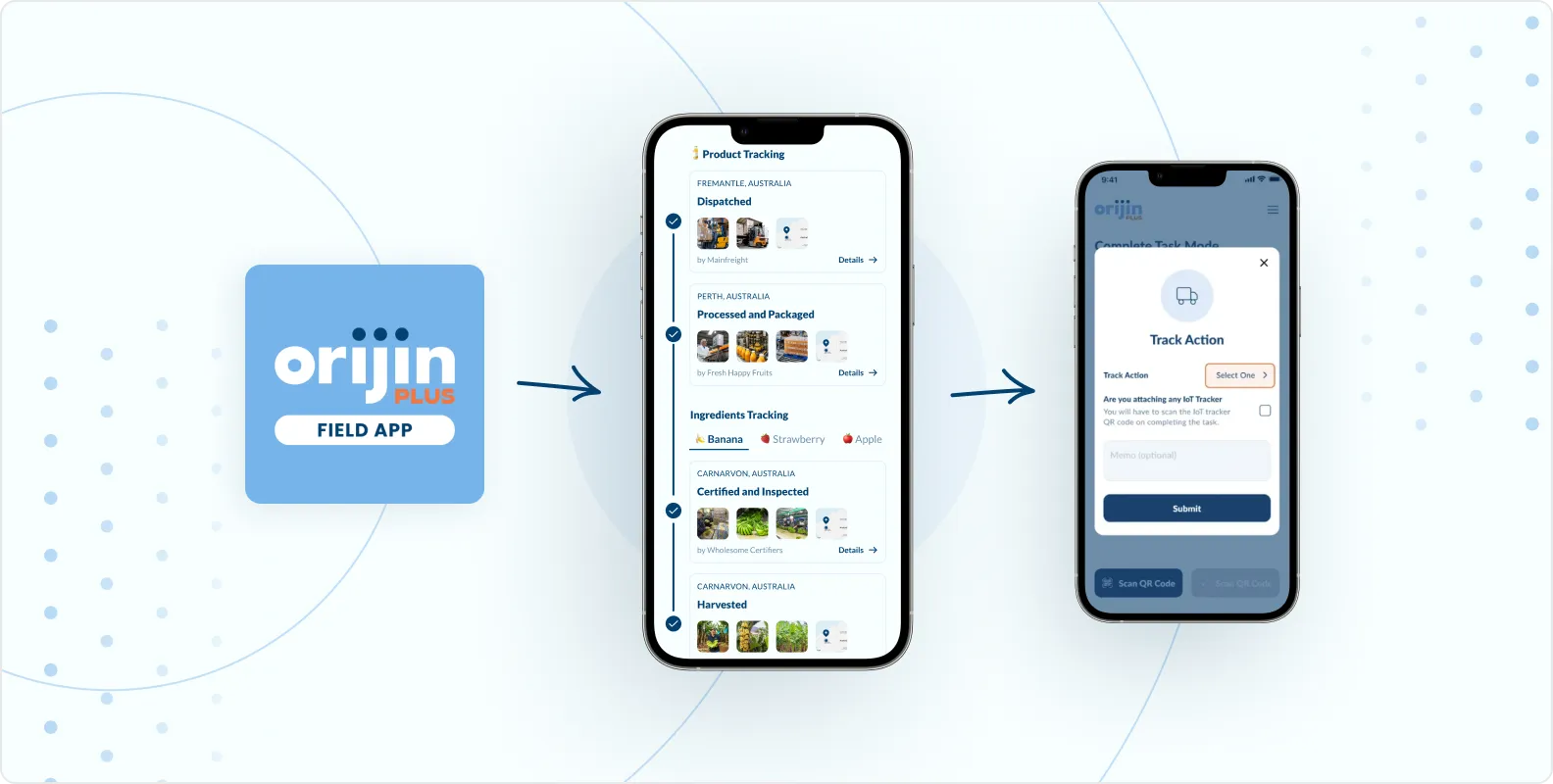
Event-based traceability aligned to FDA expectations
Orijin Plus enables traceability data to be captured and connected across Critical Tracking Events, including harvesting, receiving, transformation, and shipping. Records are linked through lots and transformations, maintaining continuity as products are combined, split, or processed across facilities.
24-hour record retrieval without manual reconciliation
Traceability data is stored in a structured, queryable format, allowing brands to retrieve and assemble required records quickly when requested. This reduces reliance on spreadsheets, emails, and fragmented systems when responding to FDA or partner requests.
Interoperable data sharing using GS1 identifiers
Orijin Plus operates a GS1-aligned resolver that associates traceability data with standard product and location identifiers. This enables traceability information to be shared efficiently with upstream and downstream trading partners, simplifying data exchange across the supply chain as FSMA expectations around interoperability continue to mature.
Future-ready traceability as requirements expand
As FDA guidance, enforcement practices, and industry data networks evolve, Orijin Plus allows brands to extend how traceability data is used. This includes supporting interoperable data sharing, integrating additional data sources, or participating in broader GS1-based traceability ecosystems without rebuilding core systems.